
CasNo: 41294-56-8
MF: C27H44O2
Appearance: white powder
|
Description |
Alfacalcidol is a vitamin D analog. It is important for the regulation of the bone metabolism. Alfacaldidol is a regulator of calcium and phosphorus metabolism, which helps calcium and phosphorus to be absorbed more effectively in the intestines. Thus, alfacalcidol is used to treat renal (kidney) bone diseases, rickets, osteoporosis, and patients with high or low activity of the parathyroid gland. Also, it is used to maintain normal calcium levels within the body. Alfacalcidol has significant effects on the immune system, including regulatory T cells. Alfacalcidol is furthermore used as a poultry feed additive to prevent tibial dyschondroplasia and increases phytate bioavailability. |
|
Physical and Chemical Properties |
White crystal or crystalline powder, insoluble in water and hexane, soluble in methanol, ethanol, chloroform, methylene chloride, acetone or ether. Perishable in case of air and light . |
|
Pharmacological effects |
Alfacalcidol is quickly converted by the liver into 1,25-dihydroxyvitamin D3, regulates calcium balance in the body, because of the enhancing of 1,25-dihydroxyvitamin D3 levels in vivo circulating , thereby increases the calcium and phosphorus intestinal absorption, promotes bone mineralization, decreases parathyroid hormone levels in plasma and reduces calcium resorption, relieves bone, muscle pain and improves osteoporosis and menopause, aging and steroid-induced intestinal related calcium malabsorption. Alfacalcidol increases the intestinal and renal tubular reabsorption of calcium and inhibit parathyroid hyperplasia, reduces parathyroid hormone synthesis and release, inhibits bone resorption. Promotes collagen and bone matrix protein synthesis.Regulates Calcium metabolism of muscles , promotes muscle cells differentiation, enhances muscle strength, increases neuromuscular coordination, tends to reduce falls. Suitable for the treatment of rickets and osteomalacia, renal osteodystrophy, osteoporosis and hypoparathyroidism. Youth are limited to young people with idiopathic osteoporosis and glucocorticoid-induced osteoporosis too. |
|
Pharmacodynamics |
This product after oral administration, is rapidly absorbed into the bloodstream from the gut, 25-hydroxy enzyme of the liver microsomal make 25-hydroxy of side chain compound to generate 1α, 25-(OH) 2D3, distributed in the intestine and bone and other target tissues, upon binding to the receptor, it can promote intestinal absorption of calcium, causing a series of physiological activities, such as bone salt dissolving and bone formation . Rats removed of the kidney and rats deficient in vitamin D, are given this product experiments, which shows that the level of promoting intestinal absorption of calcium, elevates serum calcium.Due to renal completely removed in rats removed of the kidney , the majority of bone resorption and bone-like cavity layer, low-calcified layer next layer increases, give this product 30d to experiment, the bone freshmen is observed. Therefore alfacalcidol promotes bone formation. Elderly patients with osteoporosis given this product ,iliac bone biopsy is performed by electronic and optical microscope to find that active osteoblasts, osteocytes, bone calcification small chamber increases, improves bone tissue. |
|
Indications |
Symptoms alfacalcidol can be used to improve chronic renal insufficiency, hypoparathyroidism, vitamin D-dependent rickets, a softening of bone disease caused by vitamin D metabolism, such as hypocalcemia, hand, foot cramps, pain , bone disease and osteoporosis embolism. |
|
Instructions |
For osteoporosis, oral: initial amount of 0.5μg/d, maintenance dose of 0.25~0.50μg, qd. Dialysis patients with chronic hypocalcemia, 0.5~3.0μg/d, divided 2 to 3 times daily. Hypoparathyroidism, 0.25~3.00μg/d, divided 2 to 3 times daily. Renal bone atrophy, the next day 0.25~3.00μg, divided 2 to 3 times daily. Vitamin D-dependent rickets, 1μg/d, divided 2 to 3 times daily. Familial hypophosphatemia, 2μg/d, divided 2 to 3 times daily. |
|
Adverse reactions |
Small amount alone is usually no more adverse reactions, long-term large doses of medication or in combination with calcium can cause hypercalcemia and hypercalciuria.patients in Long-term high doses and patients with renal impairment may be nausea, loss of appetite, dizziness, skin itching, rash, constipation, anorexia, vomiting, abdominal pain, high calcium signs ,it can be restored to normal after treatment. Vitamin D and its analogues allergies, hypercalcemia, vitamin D poisoning signs patients are banned. Early medication serum calcium levels should be determined weekly, when a stable dose, they are measured every 2 to 4 weeks, especially renal dysfunction serum calcium levels should be measured regularly. when hypercalcemia occurs, It Shall be discontinued and deal with hypercalcemia. After serum calcium returns to normal , the dose is dministered half of the last dose. Alfacalcidol combination with calcium can cause elevated serum calcium, when it combined with thiazide diuretics can cause the danger of hypercalcemia . When treated with digitalis drugs, care should be taken to develop the amount of this drug.The amount of Alfacalcidol should be increased when combined with barbiturates, anticonvulsants . |
|
Precautions |
If hypercalcemia occurs,it should be stopped taking until serum calcium returns to normal (about a week), and then press the last half dose administration. patients have Vitamin D symptoms or known allergy to vitamin D and its analogues is unfit for use. Patients Should avoid taking vitamin D drugs at the same time, so as not to cause vitamin D intoxication. |
|
Drug Interactions |
Using Alfacalcidol and magnesium formulation (magnesium oxide, magnesium carbonate, etc.) sometimes causes hypermagnesemia. Using Alfacalcidol and Cardiac preparations may cause cardiac arrhythmia. Using Alfacalcidol and vitamin D and its derivatives (calcitriol) , it is possible to cause hypercalcemia. Using Alfacalcidol and calcium , Thiazide diuretics, digitalis drugs , can cause hypercalcemia. And barbiturates, anti-seizure drugs may reduce the efficacy of the drug. Gastrointestinal absorption inhibitors can reduce the absorption of the drug. Combination with large doseas of Phosphorus compounds, can induce hyperphosphatemia. When Alfacalcidol and calcium preparations (calcium lactate, calcium carbonate, etc.) are used together, there may be hypercalcemia. |
|
Uses |
For the treatment of osteoporosis, vitamin D-dependent rickets and osteomalacia |
|
Referrence |
https://en.wikipedia.org/wiki/Alfacalcidol https://www.drugbank.ca/drugs/DB01436 http://www.mhra.gov.uk JD Ringe, H Faber, P Fahramand, E Schacht, Alfacalcidol versus plain vitamin D in the treatment of glucocorticoid/inflammation-induced osteoporosis, J. Rheumatol. Suppl., 2005, vol. 76, 33-40 |
|
Chemical Properties |
White Solid |
|
Originator |
One-Alpha ,Leo ,UK ,1978 |
|
Definition |
ChEBI: A member of the class of D3 vitamins that is calciol in which the hydrogen at the 1alpha position is replaced by a hydroxy group. It is an active metabolite of cholecalciferol, which performs important functions in reg lation of the calcium balance and the bone metabolism. |
|
Manufacturing Process |
1. Preparation of 1,4-cyclized adduct of cholesta-1,5,7-trien-β-ol and 4- phenyl-1,2,4-triazoline-3,5-dione: a solution of 400 mg of cholesta-1,5-7- trien-3β-ol in 30 ml of tetrahydrofuran is cooled with ice, and 190 mg of 4- phenyl-1,2,4-triazoline-3,5-dione is added little by little to the solution under agitation. The mixture is agitated at room temperature for 1 hour and the solvent is distilled under reduced pressure. The residue is purified by chromatography using a column packed with silica gel. Fractions eluted with ether-hexane (7:3 v/v) are collected and recrystallization from ether gives 550 mg of a 1,4-cyclized adduct of cholesta-1,5,7-trien-3β-ol and 4-phenyl- 1,2,4-triazoline-3,5-dione having a melting point of 178°C to 182°C. 2. Preparation of 1,4-cyclized adduct of cholesta-5,7-dien-3β-ol-1α-epoxide and 4-phenyl-1,2,4-triazoline-3,5-dione: 1.25 g of the 1,4-cyclized adduct of cholesta-1,5,7-trien-3β-ol and 4-phenyl-1,2,4-triazoline-3,5-dione is dissolved in 50 ml of chloroform, and 560 mg of m-chloroperbenzoic acid is added to the solution. The mixture is agitated for 20 hours at room temperature, and 200 mg of m-chloroperbenzoic acid is further added and the mixture is agitated again for 20 hours. The reaction mixture liquid is diluted with chloroform, washed with a 10% aqueous solution of potassium carbonate and dried with magnesium sulfate. Then, the solvent is distilled under reduced pressure. The residue is purified by silica gel chromatography, and first effluent fractions eluted with ether are collected, and recrystallization from methanol gives 680 g of a crystal melting at 172°C to 173°C. The second ether effluent fractions are collected, and recrystallization from methanol gives 400 mg of a 1,4-cyclized adduct of cholesta-5,7-dien-3β-ol-1α,2α-epoxide and 4-phenyl-1,2,4-triazoline-3,5-dione having a melting point of 152°C to 154°C. 3. Preparation of cholesta-5,7-diene-1α,3β-diol: a solution of 500 mg of the 1,4-cyclized adduct of cholesta-5,7-dien-3β-ol-1α,2α-epoxide and 4-phenyl- 1,2,4-triazoline-3,5-dione in 40 ml of tetrahydrofuran is added dropwise under agitation to a solution of 600 mg of lithium aluminum hydride in 30 ml of THF. Then, the reaction mixture liquid is gently refluxed and boiled for 1 hour and cooled, and a saturated aqueous solution of sodium sulfate is added to the reaction mixture to decompose excessive lithium aluminum hydride. The organic solvent layer is separated and dried, and the solvent is distilled. The residue is purified by chromatography using a column packed with silica gel. Fractions eluted with ether-hexane (7:3 v/v) are collected, and recrystallization from the methanol gives 400 mg of cholesta-5,7-diene-1α,3β- diol. 4. Preparation of 1α,3β-dihydroxyprovitamin D3: a solution of 25 mg of cholesta-5,7-diene-1α,3β-diol in 650 ml of ether is subjected to radiation of ultraviolet rays for 14 minutes in an argon gas atmosphere by passing it through a Vycor filter using a 200-W high pressure mercury lamp (Model 654A-36 manufactured by Hanobia). The solvent is distilled at room temperature under reduced pressure. This operation is repeated twice, and 50 mg of the so obtained crude product is fractionated by chromatography using a column packed with 20 g of Sephadex LH-20. The first effluent fractions eluted with chloroform-hexane (65:35 v/v) give 13.5 mg of oily 1α,3β- dihydroxyprovitamin D3. The composition exhibits a maximum ultraviolet absorption at 260 nm in an ether solution. 5. Preparation of 1α-hydroxycholecalciferol: a solution of 13.5 mg of 1α,3β- dihydroxyprovitamin D3 in 200 mi of ether is allowed to stand still in the dark at room temperature in an argon gas atmosphere for 2 weeks. During this period, the position of the maximum ultraviolet absorption is shifted from 260 nm to 264 nm, and the absorption intensity becomes 1.6 times as high as the original intensity. The solvent is distilled at room temperature under reduced pressure, and the residue is purified by chromatography using a column packed with 10 g of Sephadex LH-20. The fractions eluted with chloroformhexane (65:35 v/v) give 6.5 mg of oily 1α-hydroxycholecalciferol. |
|
Therapeutic Function |
Calcium regulator, Vitamin |
|
General Description |
Has biological properties similar to 1α-Hydroxyvitamin D2 (Cat. No. 679100). Converted to active Calcitriol (1α,25-Dihydroxyvitamin D3; Cat. No. 679101) in vivo. Inhibits the formation of nephrocalcinosis in streptozotocin-induced diabetic rats fed on low zinc diets. |
|
Clinical Use |
Vitamin D analogue: Increase serum calcium levels Suppression of PTH production |
|
Drug interactions |
Potentially hazardous interactions with other drugs Carbamazepine, fosphenytoin, phenytoin, phenobarbital and primidone may increase metabolism of alfacalcidol, necessitating larger doses than normal to produce the desired effect. |
|
Metabolism |
Alfacalcidol is hydroxylated in the liver by the enzyme vitamin D 25-hydroxylase to form the active 1,25-dihydroxycolecalciferol (calcitriol). Calcitriol is inactivated in both the kidney and the intestine, through the formation of a number of intermediates including the formation of the 1,24,25-trihydroxy derivatives. Vitamin D compounds and their metabolites are excreted mainly in the bile and faeces with only small amounts appearing in urine; there is some enterohepatic recycling but it is considered to have a negligible contribution to vitamin D status. |
InChI:InChI=1/C27H44O2/c1-18(2)8-6-9-19(3)24-13-14-25-21(10-7-15-27(24,25)5)11-12-22-16-23(28)17-26(29)20(22)4/h11-12,18-19,23-26,28-29H,4,6-10,13-17H2,1-3,5H3/b21-11+,22-12-/t19-,23+,24-,25?,26-,27-/m1/s1
The production of vitamin D3 is a pharma...
The invention discloses an improved prep...
The invention provides a refining method...
The invention discloses a method for pre...
Alfacalcidol (1α-hydroxyvitamin D3) is a...
(1S,6R)-3-deoxy-1-hydroxy-6-methoxy-3,5-cyclo-5,6-dihydrovitamin D3

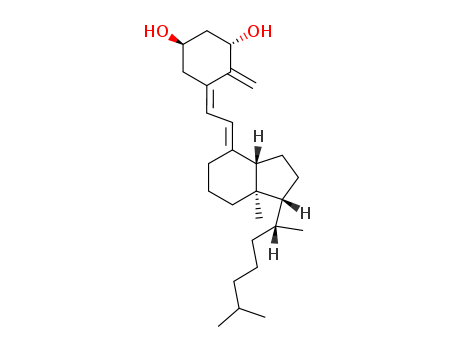
1α-hydroxyvitamin D3
| Conditions | Yield |
|---|---|
|
(1S,6R)-3-deoxy-1-hydroxy-6-methoxy-3,5-cyclo-5,6-dihydrovitamin D3; With acetic acid; In dimethyl sulfoxide; at 20 - 50 ℃; for 1h; Inert atmosphere;
With 4-Phenylurazole; In ethyl acetate; at 10 ℃; for 2h; Reagent/catalyst; Temperature; Inert atmosphere;
|
45% |
|
Multi-step reaction with 3 steps
1: Hunig base / 5 h / Ambient temperature
2: pTsOH / dioxane; H2O / 0.08 h / 55 °C
3: 39 percent / conc. HCl / methanol / 3.5 h / 60 °C
With hydrogenchloride; toluene-4-sulfonic acid; N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; methanol; water;
|
|
|
Multi-step reaction with 3 steps
1: 83 percent / N-ethyl diisopropylamine (Huenig's base) / CH2Cl2 / 1.) 0 deg C, 2.) room temperature, 5 h
2: 51 percent / water / toluene-p-sulphonic acid (PTSA) / dioxane / 0.08 h / 55 °C
3: 39 percent / conc. hydrochloric acid / methanol / 3.5 h / 60 °C
With hydrogenchloride; water; N-ethyl-N,N-diisopropylamine; toluene-4-sulfonic acid; In 1,4-dioxane; methanol; dichloromethane;
|
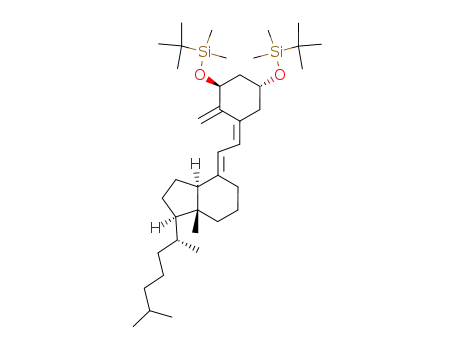
![(1R,3aS,7aR)-4-[2-[(3S,5R)-3-(tert-Butyl-dimethyl-silanyloxy)-5-(tert-butyl-diphenyl-silanyloxy)-2-methylene-cyclohex-(Z)-ylidene]-eth-(E)-ylidene]-1-((R)-1,5-dimethyl-hexyl)-7a-methyl-octahydro-indene](/upload/2024/2/3ab06a79-5619-4239-b0e4-4c80dce116b9.png)
(1R,3aS,7aR)-4-[2-[(3S,5R)-3-(tert-Butyl-dimethyl-silanyloxy)-5-(tert-butyl-diphenyl-silanyloxy)-2-methylene-cyclohex-(Z)-ylidene]-eth-(E)-ylidene]-1-((R)-1,5-dimethyl-hexyl)-7a-methyl-octahydro-indene


1α-hydroxyvitamin D3
| Conditions | Yield |
|---|---|
|
With tetrabutyl ammonium fluoride; In tetrahydrofuran; for 40h; Ambient temperature;
|
79% |
|
With tetrabutyl ammonium fluoride; In tetrahydrofuran; Ambient temperature;
|
79% |
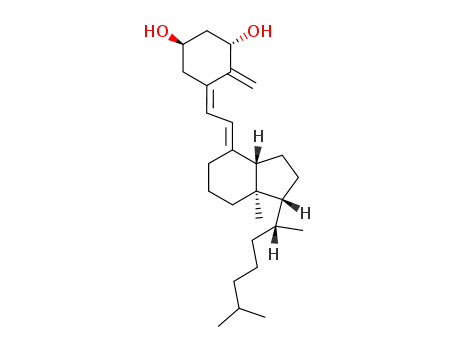
1α-hydroxyprecalciferol3
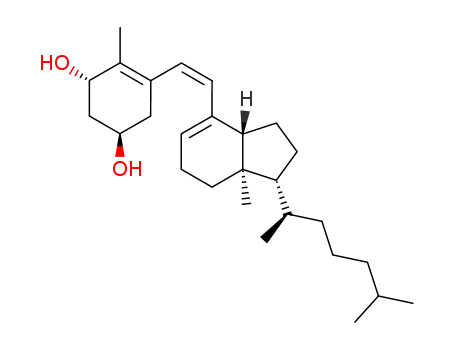
1α,3β-bis(tert-butyldimethylsilyloxy)-9,10-secocholesta-5,7,10(19)-triene
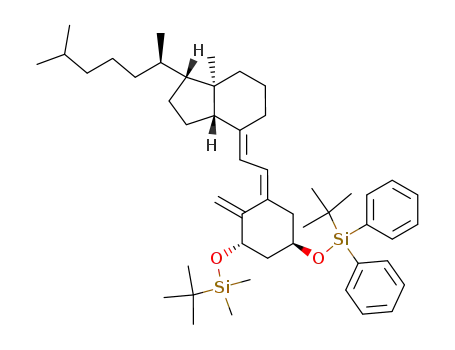
(1R,3aS,7aR)-4-[2-[(3S,5R)-3-(tert-Butyl-dimethyl-silanyloxy)-5-(tert-butyl-diphenyl-silanyloxy)-2-methylene-cyclohex-(Z)-ylidene]-eth-(E)-ylidene]-1-((R)-1,5-dimethyl-hexyl)-7a-methyl-octahydro-indene
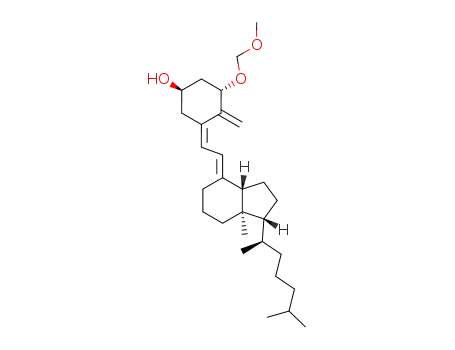
(1S)-1-methoxymethoxyvitamin D3
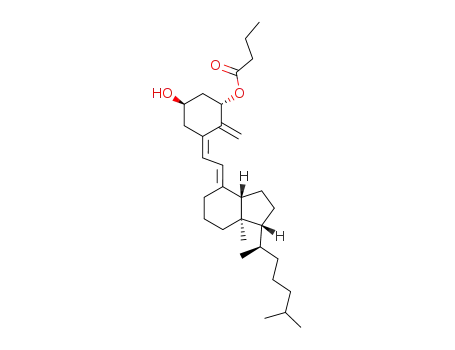
1α-Hydroxyvitamin-D3 1-butyrate

1α-hydroxyprecalciferol3
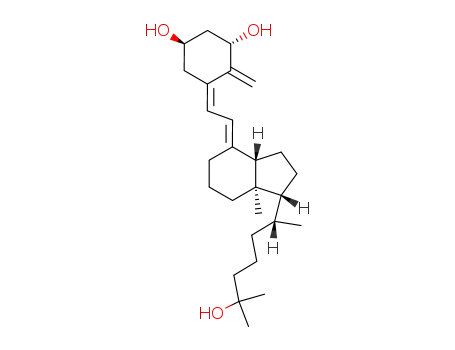
calcitriol
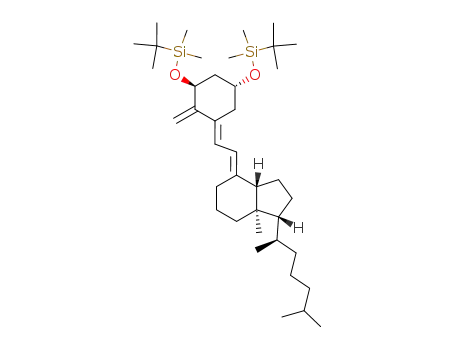
(1R,3aS,7aR)-4-[2-[(3S,5R)-3,5-Bis-(tert-butyl-dimethyl-silanyloxy)-2-methylene-cyclohex-(E)-ylidene]-eth-(E)-ylidene]-1-((R)-1,5-dimethyl-hexyl)-7a-methyl-octahydro-indene